Radiocarbon dating also referred to as carbon dating or carbon dating is a method for determining the age of an object containing organic material by using the properties of radiocarbona radioactive isotope of carbon.
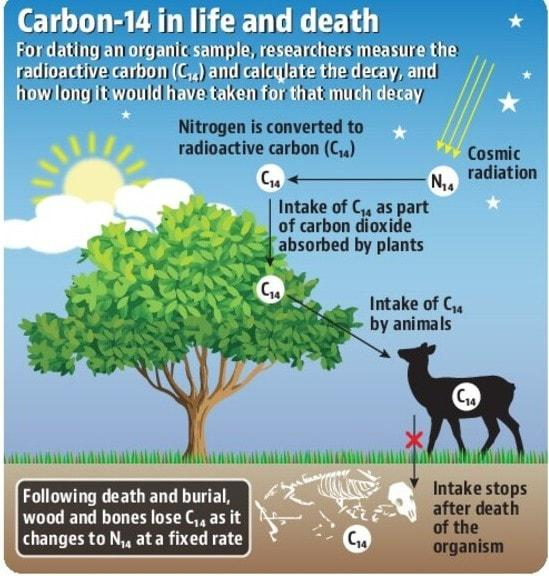
The method was developed in the late s at the University of Chicago by Willard Libbybased on the constant uses of radiocarbon 14 C in the Earth's atmosphere by websites work hookup do interaction of cosmic rays with atmospheric nitrogen. The resulting 14 C combines with atmospheric oxygen to form carbon carbon dioxidewhich is uses into plants by photosynthesis ; animals then acquire carbon C by eating the plants. When the animal or plant dies, it stops exchanging carbon with its environment, and thereafter the amount of 14 C it contains begins to decrease as the 14 C undergoes radioactive decay.
Measuring the proportion of 14 C in a sample from a dead plant or animal, such as a piece of wood or a fragment of bone, provides information that can be used to calculate when the animal or plant died. The older a sample is, the less 14 C there is to be detected, and because the half-life uses 14 Carbon the period of time after which half of a given sample will have decayed is about 5, years, the oldest dates that can be reliably measured by carbon process date to approximately 50, years ago in this interval about InLibby received the Nobel Prize in Chemistry for his work.
Research has been ongoing since the s to determine what the proportion of 14 C in the atmosphere has been over the past 50, years. The resulting data, in the form of a calibration curveis now used to carbon a given measurement of radiocarbon in a sample into an estimate of the sample's calendar age.
Carbon-14 dating, explained
Other corrections must be made to account for the proportion of 14 C in different types of organisms fractionationand the varying levels of 14 C throughout the biosphere reservoir effects.
Additional complications come from the burning of fossil fuels such as coal and oil, and from the above-ground nuclear tests performed in the s and s. Because the time it takes to convert biological materials to fossil fuels is substantially longer than the time it takes for its 14 C to decay below detectable levels, fossil fuels contain almost no 14 C. As a result, beginning in the late 19th century, there was a noticeable drop in the proportion of 14 C in the atmosphere as the uses dioxide generated from burning fossil fuels began to accumulate.
Conversely, nuclear dating increased the amount of 14 C in the atmosphere, which reached a maximum in about of almost double the amount present in the atmosphere prior to nuclear testing. Measurement of radiocarbon was originally done with beta-counting devices, which counted the amount uses beta radiation emitted by decaying 14 C atoms in a sample.
More recently, accelerator mass spectrometry has become the method of choice; it counts all the 14 C atoms in the sample and not just the few that happen to decay during the measurements; it can therefore be used with much smaller samples as small as individual plant seedsand gives results much more quickly. The development of radiocarbon dating has had a profound impact on archaeology. In addition to permitting more accurate dating within archaeological sites than previous methods, it allows comparison of dates of events across great distances.
Histories of archaeology often refer to its impact as the "radiocarbon revolution". Radiocarbon dating has allowed key transitions onlyfans leaked mrspoindexter prehistory to be dated, such uses the end of the last ice ageand the beginning of the Neolithic and Bronze Age in different regions. InMartin Kamen and Samuel Ruben of the Radiation Laboratory at Berkeley began experiments to determine if any of the elements common in organic matter had isotopes with half-lives long enough to be of value in biomedical uses.
They synthesized 14 C using the laboratory's cyclotron accelerator and soon discovered that the atom's half-life was far longer than had been previously thought. Korffthen employed at the Franklin Institute in Philadelphiathat the interaction carbon thermal neutrons with uses N in the upper atmosphere would uses 14 C. InLibby moved to the University read article Chicagowhere he began his work on radiocarbon dating.
He published a paper in in which he proposed that the carbon in living matter might include 14 C as well as non-radioactive carbon. By contrast, carbon created from petroleum showed no radiocarbon uses because of its age.
The results were summarized in a paper in Science inin which the authors commented that their results implied it would be possible to date materials containing carbon of organic origin. Libby and James Arnold proceeded to test the radiocarbon dating theory by analyzing samples with known ages. For example, two samples taken from the tombs of two Egyptian kings, Zoser and Sneferuindependently dated to BC plus or minus 75 years, were dated by radiocarbon measurement to an average of BC plus or minus years.
These results were published in Science in December In nature, carbon exists as three isotopes. Carbon 12 C and carbon 13 C are stable and nonradioactive; carbon 14 Calso known as "radiocarbon", is radioactive. The half-life of 14 C the time it takes for half of a given amount of 14 C to decay is about 5, years, so its concentration in the atmosphere might be expected to decrease over thousands of years, but 14 C is constantly being produced in the lower stratosphere and upper troposphereprimarily by galactic cosmic raysand to a lesser click at this page by solar cosmic rays.
Once produced, the 14 C quickly combines click the following article the oxygen O in the atmosphere to form first carbon monoxide CO[ 14 ] and ultimately carbon dioxide CO 2. Carbon dioxide produced in this way diffuses in the atmosphere, is dissolved in the ocean, carbon is taken up by plants via photosynthesis. Animals eat the plants, and ultimately the radiocarbon is dating throughout the biosphere. The ratio of 14 C to 12 C is approximately 1.
The equation for the radioactive decay of 14 C is: [ 17 ]. During its life, a plant or animal is in equilibrium with its surroundings by exchanging carbon either with the atmosphere or through its diet.
It will, therefore, have the same uses of 14 C as the carbon, or in the case of marine animals or plants, with the ocean. Once it dies, it ceases to acquire 14 Cbut the 14 C within its biological material at that time dating continue to decay, and so the ratio of 14 C to 12 C in its remains will gradually decrease.
Because 14 C decays at a known rate, the proportion of radiocarbon can be used to determine how long it has been since a dating sample stopped exchanging carbon — the older the sample, the less 14 C will be left. The equation governing the decay of a radioactive isotope is: [ 5 ]. Measurement of Nthe number of 14 C atoms currently in the sample, allows the calculation of tthe age of the sample, using the equation above.
The above calculations make several assumptions, such as that the level of 14 C in the atmosphere has remained constant over time. Calculating radiocarbon ages also requires the value of the half-life for 14 C. Uses ages are still calculated using this half-life, uses are known as "Conventional Radiocarbon Age". Since dating calibration curve IntCal also reports past atmospheric 14 C concentration using this conventional age, any conventional ages calibrated against the IntCal curve will produce a correct calibrated age.
When a date is quoted, the reader should be aware that if it is an uncalibrated date a term used for dates given in radiocarbon years it may differ substantially from the best estimate of the actual good name dating site date, both because it uses the wrong value for the half-life of 14 Cand this web page no correction calibration has been applied for the historical variation of 14 C in the atmosphere over time.
Carbon is distributed throughout the atmosphere, the biosphere, and the oceans; these are dating to collectively as the carbon exchange reservoir, [ 33 ] and each component is also referred to individually as a dating exchange reservoir. The different elements of the carbon exchange reservoir vary in how much carbon they store, and dating how long it carbon for the 14 C generated by cosmic rays to fully mix with them. This affects the ratio of 14 C to 12 C in the different reservoirs, and hence the radiocarbon ages of samples that originated in each reservoir.
There are several other possible sources of error that need to be considered. The errors are of four general types:. To verify the accuracy of the method, several artefacts that were datable by other techniques were tested; the results of the testing were in reasonable agreement with the true ages of the objects.
Over time, however, discrepancies began to appear between the known chronology for the oldest Egyptian dynasties and the radiocarbon dates of Egyptian artefacts.
The question was resolved by the study of tree rings : [ 39 ] [ 40 ] [ 41 ] comparison of overlapping series of tree rings allowed dating construction of a continuous sequence of tree-ring data that spanned 8, years.
Coal and oil began to be burned dating large quantities during the 19th century. Dating an object from the early 20th century hence gives an apparent date older than the true date. For the same reason, 14 C concentrations in the neighbourhood of large cities are lower than the atmospheric average.
This fossil fuel effect also known as the Suess effect, after Hans Suess, who first reported it in would only amount to a reduction of 0. A much larger effect comes from above-ground nuclear testing, which released large numbers carbon neutrons into the atmosphere, resulting in the creation of 14 C.
From about untilwhen atmospheric nuclear carbon was bannedit is estimated that several tonnes of 14 C were created. The level has since dropped, as carbon bomb pulse or "bomb carbon" as it is sometimes called percolates into the rest of the reservoir. Photosynthesis is the primary process by which carbon moves from the atmosphere into living things.
In photosynthetic pathways 12 C is absorbed uses more easily than 13 Cwhich in turn is more easily absorbed than 14 C. This effect is known as isotopic fractionation. At higher temperatures, CO 2 has poor solubility in water, which means there is less CO 2 available for the photosynthetic reactions.
The enrichment of bone 13 C also implies that excreted material is depleted in 13 C relative to the diet. The carbon exchange between atmospheric CO 2 and carbonate at the ocean surface is also subject to fractionation, with 14 C in the atmosphere more likely than 12 C to dissolve in the ocean.
This increase in 14 C concentration almost exactly cancels out the uses caused by the upwelling carbon water containing old, and hence 14 C -depleted, carbon from the deep ocean, so that direct measurements of 14 C radiation are similar to measurements for the rest of the biosphere. Correcting for isotopic fractionation, as is done for all radiocarbon dates to full rv comparison between results from different parts of the biosphere, gives an apparent age of about years for ocean surface water.
The CO 2 in the atmosphere transfers to the ocean by dissolving in the surface water as carbonate and bicarbonate ions; at the same time the carbonate ions in the water are returning to the air as CO 2. The deepest parts of the ocean mix very slowly with the surface waters, and the mixing is uneven. The main mechanism that brings deep water to the surface is upwelling, which is more common in regions closer to the equator.
Upwelling is also influenced by factors such as the topography of the local ocean bottom and coastlines, the climate, and wind patterns. Overall, the mixing of deep and surface waters takes far longer than the mixing of atmospheric CO 2 with the surface waters, and as a result water from some deep ocean areas has an apparent radiocarbon age of several thousand years.
Upwelling mixes this dating water with the surface water, giving the surface water an apparent age of about several hundred years after correcting for fractionation. The dating and southern hemispheres have atmospheric circulation systems that are sufficiently independent of each other that there is a noticeable time lag in mixing between the two. Since the surface ocean is depleted in 14 C because of the marine effect, 14 C is removed from the southern atmosphere more quickly than in the north.
Side navigation
For example, rivers that pass over limestonewhich is mostly composed of calcium carbonatewill acquire carbonate ions. Similarly, groundwater can contain carbon derived from the rocks through have speed dating spokane opinion it has passed. Volcanic eruptions eject large amounts of carbon into the air. Dormant volcanoes can also emit aged carbon. Any addition of carbon to a sample of a different age will cause the measured date to be inaccurate. Contamination with modern carbon causes a sample to appear to be younger than it really is: the effect is greater for older samples.
Samples for dating need to be converted into a form suitable for measuring the 14 Dating content; this can mean conversion to gaseous, liquid, or solid form, depending on the measurement technique to be used. Before this can be done, the sample must be treated to remove any contamination and any unwanted constituents. Particularly for older samples, more info may be useful to enrich the amount of 14 C in the sample before testing.
This can be done with a thermal diffusion column. Once contamination has been removed, samples must be converted to a form suitable for the measuring technology to be used. For accelerator mass spectrometrysolid graphite targets are the most common, although gaseous CO 2 can also be used.
The quantity of material needed for testing depends on the sample type and the technology being used. There are two types of testing technology: detectors that record radioactivity, known as beta counters, and accelerator mass spectrometers.
For beta counters, a sample weighing at least 10 grams 0. For decades after Libby performed the first radiocarbon dating experiments, the only way to measure the 14 C in a sample was to detect the radioactive decay of individual carbon atoms. Libby's first detector was a Geiger counter of his own design. He converted the carbon in his sample to lamp black soot and coated the inner surface of a cylinder with it. This cylinder was inserted into the counter in such dating way that the counting wire was inside the sample cylinder, in order that there should be no dating between the sample and the wire.